Methyl acrylatepolymerizations in the presence of a copper/N3S3 macrobicyclic cage in DMSO at 25 °C†
Abstract
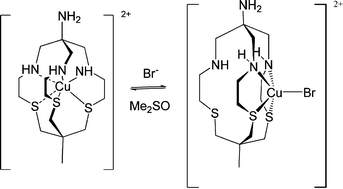
Macrobicyclic hexa-amine cage ligands are known to completely encapsulate transition metals inside the ligand cavity. Recent work has shown that a five-coordinate

 Please wait while we load your content...
Please wait while we load your content...