Modulation of the carboxamidine redox potential through photoinduced spiropyran or fulgimide isomerisation†
Abstract
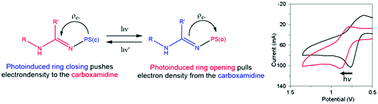
Carboxamidines functionalized with either a spiropyran or fulgimide photoswitch were prepared on multigram scales. The thermal, electrochemical, and photochemical ring isomerizations of these compounds were studied and the results compared with related systems. The photochemical isomerisations were found to be reversible and could be followed by 1H NMR and UV-vis spectroscopy. The spiropyran/merocyanine couple was thermally active and an activation enthalpy of 116 kJ mol−1 was measured for ring-opening. These measurements yielded an enthalpy difference of 25 kJ mol−1 between the open and closed states which is consistent with DFT calculations. DFT calculations predicted a charge transfer to the carboxamidine group upon ring closure in the fulgimide and a charge transfer from the carboxamidine group upon switching the spiropyran to the merocyanine form. This was confirmed experimentally by monitoring the change in the oxidation potential assigned to the carboxamidine group. The potential of these molecules to therefore act as a new class of photoresponsive ligands that can modulate the ligand field of a complex is discussed.


 Please wait while we load your content...
Please wait while we load your content...
