Fluorescence properties of aurone derivatives: an experimental and theoretical study with some preliminary biological applications†
Abstract
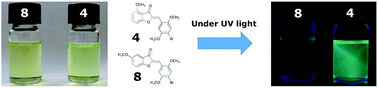
In this paper, we explored the fluorescence properties of eight aurone derivatives bearing methoxy groups and bromine atoms as substituents in the benzene rings. All derivatives showed strong solvatochromic absorption and emission properties in solvents of different polarities. Some of them showed high fluorescence quantum yields, which make them potential compounds for sensing applications. The position of the methoxy groups in the benzofuranone moiety and the presence of bromine atoms in the benzene ring had a strong influence on the fluorescence behaviour of the aurones. DFT calculations allowed us to explain the emission properties of aurones and their solvatochromism, which was related to an excited state with strong charge-transfer character. Aurone 4 has the most promising characteristics showing a large difference in the quantum yields and large Stokes shifts depending on the solvent polarities. These results prompted us to explore some preliminary biological applications for aurone 4 such as the sensing of hydrophobic pockets of a protein and its thermotropic behaviour in liposomes.


 Please wait while we load your content...
Please wait while we load your content...