Benzoylformamides as versatile photocaged bases for redox free radical photopolymerization
Abstract
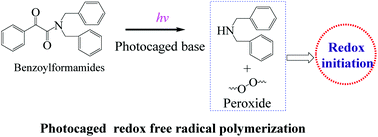
Benzoylformamides (BFAs) are proposed as photocaged bases for redox free radical photopolymerization (FRP). Initially the dissolving capacity of BFAs was evaluated. Besides our previously reported photo-latent anion polymerization, BFAs, as versatile photocaged bases, can not only initiate the FRP of acrylates unassisted, but also perform redox FRP in combination with a benzoyl peroxide initiator. We detail a model photopolymerization in the presence of BFA-dBA (N,N-dibenzyl-2-oxo-2-phenylacetamide) as the photocaged base. In combination with a benzoyl peroxide initiator, BFAs are able to initiate the amine-mediated redox photopolymerization of acrylates, and enhance the photopolymerization rate. Finally, we investigated the mechanism of the radical formation to initiate the redox FRP, as exemplified by BFA-dBA.

 Please wait while we load your content...
Please wait while we load your content...