New insight into the photophysics and reactivity of trigonal and tetrahedral arylboron compounds†
Abstract
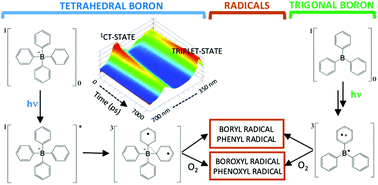
The photophysics and reactivity of two tetraphenylborate salts and triphenylborane have been studied using ultrafast transient absorption, steady-state fluorescence, electron paramagnetic resonance with spin trapping, and DFT calculations. The singlet excited state of tetraarylborates exhibit extended π-orbital coupling between two adjacent aryl groups. The maximum fluorescence band, as well as the transient absorption bands centered at 560 nm (τ = 1.05 ns) and 680 nm (τ = 4.35 ns) are influenced by solvent viscosity and polarity, indicative of a twisted intramolecular charge transfer (TICT) state. Orbital contour plots of the HOMO and LUMO orbitals of the tetraarylboron compounds support the existence of electron delocalization between two aryl groups in the LUMO. This TICT-state and aryl–aryl electron extension is not observed for the trigonal arylboron compound, in which excited π-orbital coupling only occurs between the boron atom and one aryl group, which restricts the twist motion of the aryl–boron bond. The excited triplet state is deactivated primarily through aryl–boron bond cleavage, yielding aryl and diphenylboryl radicals. In the presence of oxygen, this photochemistry results in phenoxyl and diphenylboroxyl radicals, as confirmed by EPR spectroscopy of spin trapped radical adducts. The TICT transition and radical generation is not expected for BoDIPY molecules where the rotational vibration of the B–aryl bond is rigid, restricting changes in the geometric structure. In this sense, this work contributes to the development of new BoDIPY derivatives where the TICT transition may be observed for aryl ligands with free rotational vibrations in the BoDIPY structure.

 Please wait while we load your content...
Please wait while we load your content...