The dark and bright sides of an enzyme: a three dimensional structure of the N-terminal domain of Zophobas morio luciferase-like enzyme, inferences on the biological function and origin of oxygenase/luciferase activity
Abstract
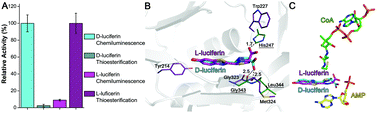
Beetle luciferases, the enzymes responsible for bioluminescence, are special cases of CoA-ligases which have acquired a novel oxygenase activity, offering elegant models to investigate the structural origin of novel catalytic functions in enzymes. What the original function of their ancestors was, and how the new oxygenase function emerged leading to bioluminescence remains unclear. To address these questions, we solved the crystal structure of a recently cloned Malpighian luciferase-like enzyme of unknown function from Zophobas morio mealworms, which displays weak luminescence with ATP and the xenobiotic firefly D-luciferin. The three dimensional structure of the N-terminal domain showed the expected general fold of CoA-ligases, with a unique carboxylic substrate binding pocket, permitting the binding and CoA-thioesterification activity with a broad range of carboxylic substrates, including short-, medium-chain and aromatic acids, indicating a generalist function consistent with a xenobiotic-ligase. The thioesterification activity with L-luciferin, but not with the D-enantiomer, confirms that the oxygenase activity emerged from a stereoselective impediment of the thioesterification reaction with the latter, favoring the alternative chemiluminescence oxidative reaction. The structure and site-directed mutagenesis support the involvement of the main-chain amide carbonyl of the invariant glycine G323 as the catalytic base for luciferin C4 proton abstraction during the oxygenase activity in this enzyme and in beetle luciferases (G343).

 Please wait while we load your content...
Please wait while we load your content...