Preparation and photophysical properties of fluorescent difluoroboronated β-diketones having phenanthrene moieties studied by emission and transient absorption measurements†
Abstract
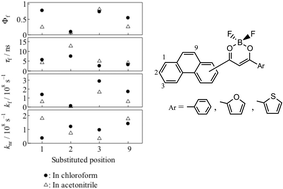
Six difluoroboronated β-diketones having the phenanthrene skeleton (Phe@Ar) are prepared. Based on the measurements of the fluorescence quantum yields, lifetimes and transient absorption, the photophysical features of Phe@Ar are studied in comparison with those of difluoroboronated diketones having phenyl, naphthyl and anthryl moieties. β-Diketones having 1-, 2-, 3- and 9-phenanthryl moieties (PheDKAr) were prepared as the precursor to Phe@Ar. 1-Acetylphenanthrene was synthesized by the photocyclization method as the key building block of PheDKAr having the 1-phenanthryl moiety. The counter aromatic moieties (Ar) of the prepared PheDKAr are varied with phenyl, furyl and thienyl rings (Ar = Ph, F and T, respectively) to investigate the effects of π-conjugation on the fluorescence properties. The prepared Phe@Ars are fluorescent with appreciable fluorescence quantum yields which depend on the substitution position of the phenanthrene moiety. 3-Phe@Ph having the 3-phenanthryl moiety provides the largest fluorescence quantum yield (0.81) in acetonitrile among the Phe@Ars whereas 2-Phe@Ph having the 2-phenanthryl moiety shows the smallest fluorescence quantum yield (0.07) in acetonitrile. All the Phe@Ars show fluorescence also in the solid state, and the fluorescence spectra and quantum yields were determined. Transient absorption measurement using laser flash photolysis of the Phe@Ars revealed the triplet formation. DFT and TD-DFT calculations of Phe@Ars rationalize the dependency of the fluorescence quantum yields on the substitution position of the phenanthrene skeleton in terms of difference in the oscillator strength for the HOMO–LUMO transition.

 Please wait while we load your content...
Please wait while we load your content...