Photoactivatable, biologically-relevant phenols with sensitivity toward 2-photon excitation†
Abstract
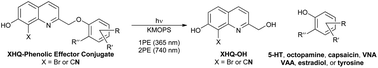
Spatio-temporal release of biologically relevant small molecules provides exquisite control over the activation of receptors and signaling pathways. This can be accomplished via a photochemical reaction that releases the desired small molecule in response to irradiation with light. A series of biologically-relevant signaling molecules (serotonin, octopamine, capsaicin, N-vanillyl-nonanoylamide, estradiol, and tyrosine) that contain a phenol moiety were conjugated to the 8-bromo-7-hydroxyquinolinyl (BHQ) or 8-cyano-7-hydroxyquinolinyl (CyHQ) photoremovable protecting groups (PPGs). The CyHQ caged compounds proved sensitive toward 1PE and 2PE processes with quantum efficiencies of 0.2–0.4 upon irradiation at 365 nm and two-photon action cross sections of 0.15–0.31 GM when irradiated at 740 nm. All but one BHQ caged compound, BHQ-estradiol, were found to be sensitive to photolysis through 1PE and 2PE with quantum efficiencies of 0.30–0.40 and two photon cross sections of 0.40–0.60 GM. Instead of releasing estradiol, BHQ-estradiol underwent debromination.

 Please wait while we load your content...
Please wait while we load your content...