Neutral and cationic pyridylbutadienes: solvatochromism and fluorescence response with sodium cholate†
Abstract
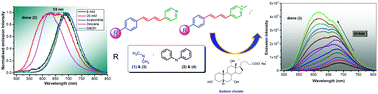
Neutral and cationic diarylbutadienes with pyridine and pyridinium groups as electron acceptors with dimethylamine and diphenylamine as electron donors were synthesized. The absorption and emission properties of these dienes were investigated in homogeneous organic solvents and in sodium cholate media. In strongly polar solvents, both neutral and cationic fluorophores exhibit a bathochromic shift of emission characteristics of intramolecular charge transfer (ICT) excited states. The presence of a strongly electron withdrawing pyridinium group induces large absorption (∼480 nm) and emission wavelength shifts (near ∼680 nm) in polar solvents. This solvatochromic behavior is utilized as a tool to examine the fluorophore–sodium cholate interaction. Significant hypsochromic emission shifts (up to ∼66 nm) and concomitant intensity enhancement (up to 32-fold) are seen for these fluorophores in the presence of increasing concentrations of sodium cholate. Fluorescence lifetime measurements reveal biexponential decay patterns indicating the binding of molecules in distinct cholate environments.

 Please wait while we load your content...
Please wait while we load your content...