Spectral fine tuning of cyanine dyes: electron donor–acceptor substituted analogues of thiazole orange†
Abstract
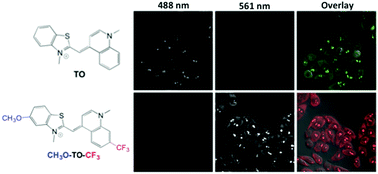
The introduction of electron donor and acceptor groups at strategic locations on a fluorogenic cyanine dye allows fine-tuning of the absorption and emission spectra while preserving the ability of the dye to bind to biomolecular hosts such as double-stranded DNA and a single-chain antibody fragment originally selected for binding to the parent unsubstituted dye, thiazole orange (TO). The observed spectral shifts are consistent with calculated HOMO–LUMO energy gaps and reflect electron density localization on the quinoline half of TO in the LUMO. A dye bearing donating methoxy and withdrawing trifluoromethyl groups on the benzothiazole and quinoline rings, respectively, shifts the absorption spectrum to sufficiently longer wavelengths to allow excitation at green wavelengths as opposed to the parent dye, which is optimally excited in the blue.

 Please wait while we load your content...
Please wait while we load your content...