Effects of iptycene scaffolds on the photoluminescence of N,N-dimethylaminobenzonitrile and its analogues†‡
Abstract
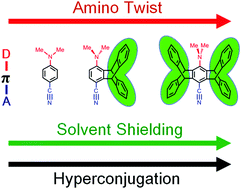
To understand the effect of iptycene scaffolds on the locally excited (LE) and intramolecular charge transfer (ICT) fluorescence of aminobenzonitriles, a series of triptycene and pentiptycene derivatives were synthesized and their molecular structures and photophysical properties were characterized and compared with the parent phenylene systems, 4-(N-methylamino)benzonitrile (MABN), 4-(N,N-dimethylamino)benzonitrile (DMABN), and 4-(N-phenylamino)benzonitrile (PABN). The iptycene effect does not change the nature of the fluorescing states for each amino donor system, i.e., the MA, PA, and DMA series display LE-only, ICT-only, and LE-ICT dual fluorescences, respectively. However, the iptycene scaffolds impose a significant modification of the absorption and emission spectra, fluorescence quantum efficiency and lifetimes, and the interplay of LE and ICT states. The observed iptycene effect has been discussed with three factors: (1) steric effect on increasing the amino twist angle, (2) steric shielding of solvation to the aminobenzonitrile core, and (3) hyperconjugation interactions of the aminobenzonitrile core with the peripheral phenylene groups of iptycene.
- This article is part of the themed collection: Dedicated to the memory of Prof. Nicholas J. Turro

 Please wait while we load your content...
Please wait while we load your content...