Iodine-sensitized oxidation of ferrous ions under UV and visible light: the influencing factors and reaction mechanism
Abstract
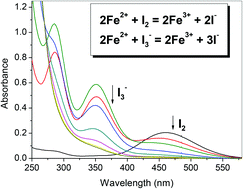
The sensitized oxidation of Fe2+ ions by I2 or I3− in acidic aqueous solution has been examined. The reactions could occur either under UV or visible light, with the stoichiometric formation of Fe3+ and I−. However, the I3−-sensitized reaction was fast and complete only in the presence of excess Fe2+. Through a kinetics study, it becomes clear that I˙ and I2−˙ are the main reactive species for the I2 and I3−- sensitized oxidation of Fe2+, respectively. Moreover, the quantum yields determined with the I2 and I3−-sensitized formation of Fe3+ at 456 nm were 0.119 and 0.118, respectively. The I2-sensitized reaction was first order only in I2, and had an Arrhenius activation energy of 29.3 kJ mol−1. It is proposed that the process of Fe2+ oxidation by I˙ is fast, while the rate-determining step is the formation and self-recombination of I˙ radicals.

 Please wait while we load your content...
Please wait while we load your content...