Visible-light-induced, copper(i)-catalysed C–N coupling between o-phenylenediamine and terminal alkynes: one-pot synthesis of 3-phenyl-2-hydroxy-quinoxalines†‡
Abstract
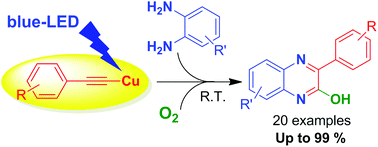
Visible-light-initiated aerobic direct C–N coupling between o-phenylenediamines and terminal acetylenes was performed using simple copper(I) chloride as a catalyst for the synthesis of quinoxaline derivatives. The current method works well for a wide range of electron rich as well as electron poor group-substituted o-phenylenediamines and phenylacetylenes. The key component in the reaction is the direct photo-excitation of in situ generated copper arylacetylide (λabs = 420–480 nm). Moreover, as compared to the literature reports (thermal process), the current photochemical method is simple, mild, high yielding, and more viable towards the construction of biologically important quinoxaline derivatives from easily accessible raw materials, without the need of ligands and strong oxidants.

 Please wait while we load your content...
Please wait while we load your content...