UV-induced formation of the thymine–thymine pyrimidine (6-4) pyrimidone photoproduct – a DFT study of the oxetane intermediate ring opening†
Abstract
The mechanism by which the hypothetical
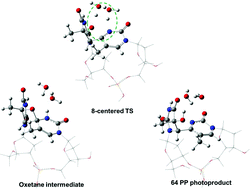
- This article is part of the themed collection: Interaction of UV radiation with DNA

 Please wait while we load your content...
Please wait while we load your content...