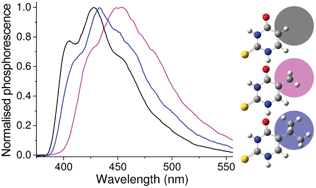
The aim of the present work is to determine the influence of C5 substitution on the photophysical properties of 2-thiopyrimidines (2-TPyr). For this purpose, 2-thiouracil, 5-t-butyl-2-thiouracil and 2-thiothymine (TU, BTU and TT, respectively) have been selected as target thionucleobases for the experimental studies and, in parallel, for DFT theoretical calculations. The UV spectra displayed by TU, BTU and TT in EtOH were very similar to each other. They showed a maximum around 275 nm and a shoulder at ca. 290 nm. The three 2-TPyr exhibited a strong phosphorescence emission; from the recorded spectra, triplet excited state energies of ca. 307, 304 and 294 kJ mol−1 were determined for TU, BTU and TT, respectively. Laser excitation at 308 nm gave rise to a broad transient absorption band from 500 nm to 700 nm, which was in principle assigned to triplet–triplet absorption. This assignment was confirmed by energy transfer experiments using biphenyl (ET = 274 kJ mol−1) as an acceptor. The triplet lifetimes were 70 ns, 1.1 μs and 2.3 μs, for TU, BTU and TT, respectively. The obtained photophysical data, both in phosphorescence and transient absorption measurements, point to significantly different properties of the TT triplet excited state in spite of the structural similarities. Theoretical calculations at the B3LYP/aug-cc-pVDZ/PCM level agree well with the experimental range of excited state energies and support the ππ* nature of the lowest triplet states.

 Please wait while we load your content...
Please wait while we load your content...