Photophysics and halide quenching of a cationic metalloporphyrin in water†
Abstract
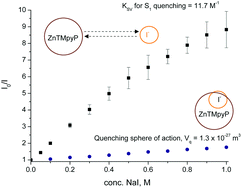
In this work, the steady state S0–S1 and S0–S2 absorption and emission behaviour of the water-soluble tetrakis(N-methyl-4-pyridyl)porphyrin zinc(II) tetrachloride (ZnTMPyP) in media of constant and high ionic strength, both with and without iodide ions as a fluorescence quencher, was measured. The quenching of the ZnTMPyP S1 state by iodide ions proceeds primarily through diffusion-limited interaction in an encounter pair but the formation of a loose association between the ZnTMPyP S1 state and iodide ions also provides a minor quenching pathway. The ZnTMPyP S2 state was quenched minimally by iodide, likely through an electron transfer mechanism at an average donor–acceptor distance of ∼0.7 nm. The results presented here highlight the notion that significant iodide quenching of the ZnTMPyP S1 state can be a source of inefficiencies in

 Please wait while we load your content...
Please wait while we load your content...