Fluorescence and two-photon absorption of push–pull aryl(bi)thiophenes: structure–property relationships†‡
Abstract
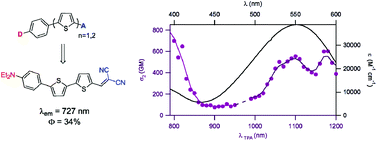
Photophysical and TPA properties of series of push–pull aryl(bi)thiophene
- This article is part of the themed collection: In honour of Jean-Pierre Desvergne

 Please wait while we load your content...
Please wait while we load your content...