Kinetic and analytical study of the photo-induced degradation of monuron by nitrates and nitrites under irradiation or in the dark
Abstract
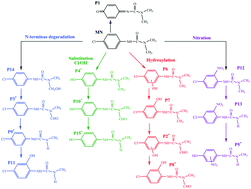
The photo-induced transformation of monuron (3-(4-chlorophenyl)-1,1 dimethylurea) was investigated in an aqueous solution containing nitrates and nitrites at 310 nm and 365 nm, respectively. In both NO3− and NO2− conditions, the degradation of monuron followed pseudo-first order kinetics. The intermediate products were identified by GC-MS, and the nitration, hydroxylation and coupling reactions were determined. In addition, the oxidation of the N-terminus group, the substitution of chlorine by ˙OH and the nitration by ˙NO2 radical onto the phenyl ring were observed. The photo-induced transformation of monuron was studied under variable conditions of pH, inducer concentration, substrate concentration, humic acids, oxygen content and salts used as hydroxyl radical scavengers. The photodegradation rates were strongly influenced by all the above parameters. The degradation of monuron was also studied in the dark and in the presence of NO2− as well as in an aqueous solution with the addition of hydrogen peroxide.

 Please wait while we load your content...
Please wait while we load your content...