The donor–acceptor biphenyl platform: A versatile chromophore for the engineering of highly efficient two-photon sensitive photoremovable protecting groups†
Abstract
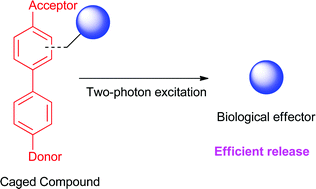
Different photoremovable protecting groups in the o-nitrobenzyl, phenacyl, and 2-(o-nitrophenyl)propyl series with a donor–acceptor biphenyl backbone, known to display excellent two-
- This article is part of the themed collection: Photoremovable protecting groups: development and applications

 Please wait while we load your content...
Please wait while we load your content...