Photophysics and reductive quenching reactivity of gadusol in solution
Abstract
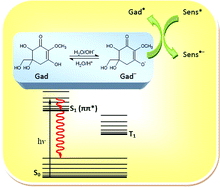
The photostability and photophysics of gadusol in aqueous solution has been studied. The photodecomposition quantum yields (ca. 4 × 10−2 and 1 × 10−4 at acidic and neutral pH, respectively) confirm the high photostability of the

 Please wait while we load your content...
Please wait while we load your content...