Bromines on N-allyl position of cationic porphyrins affect both radio- and photosensitizing properties
Abstract
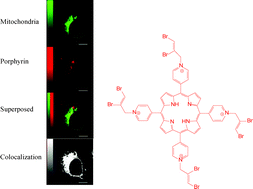
With the aim to develop improved dual-action sensitizers suitable for both photodynamic therapy (PDT) and radiotherapy, we prepared a series of metal and metal-free cationic

 Please wait while we load your content...
Please wait while we load your content...