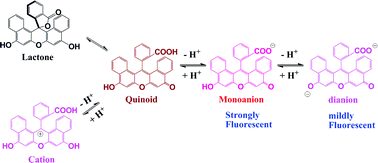
The protolytic equilibria of 1,2,7,8-dibenzofluorescein in aqueous solution have been characterized by visible absorption and fluorescence spectra. The species involved are identified as dianion, monoanion, neutral form and cation. The neutral form includes both the quinoid and lactone structures. The pKas were calculated by an improved procedure to be 3.14, 4.04 and 6.28, respectively. The absorption spectra for each protolytic form were resolved. The absorption maxima (molar absorption coefficient, ×105, M−1 cm−1) are 532 nm (0.87) for the dianion, 510 nm (0.39) for the monoanion, 500 nm (0.16) for the neutral form, and 494 nm (0.19) for the cation, respectively. Contrary to the assumption in the literature, we found that the monoanion is highly fluorescent (Φf = 0.66, compared to Φf = 0.25 for dianion) and its molar ratio can reach 50% at neutral pH. It is therefore concluded that under physiological pH conditions the monoanion plays a major role when it is used as a fluorescence probe.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...