Early molecular events in the photoactive yellow protein: role of the chromophore photophysics
Abstract
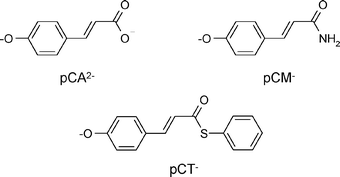
We report a comparative study of the isomerization reaction in native and denatured photoactive yellow
- This article is part of the themed collection: 10th Congress of the European Society for Photobiology

 Please wait while we load your content...
Please wait while we load your content...