Photocatalytic degradation of 4-chlorophenoxyacetic acid in the presence of an iron complex and hydrogen peroxide
Abstract
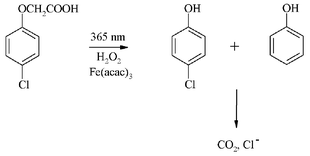
The photoinitiated transformation of 4-chlorophenoxyacetic acid (CPA) in aqueous solutions is described in detail. The photocatalytic system consists of CPA, hydrogen peroxide (no oxygen) and the complex iron(III) acetylacetonate Fe(acac)3. Special attention was paid to the choice of irradiation wavelength in order to separate the contribution of the iron-photocatalyzed reaction from that of direct photoreactions. This was achieved by selecting a wavelength of 365 nm, which is absorbed exclusively by the iron complex. We specified the conditions for effective photocatalytic degradation of CPA with high quantum yields which depend on the concentration of Fe(acac)3. The reaction is primarily initiated by absorption of 365 nm radiation by Fe(acac)3 followed by the photoreduction of Fe(III)(acac)3 to Fe(II)(acac)2 and subsequently by the Fenton reaction with hydrogen peroxide producing highly reactive hydroxyl radicals OH˙. The main degradation intermediates are phenol and 4-chlorophenol, which are further mineralized during continuous irradiation.

 Please wait while we load your content...
Please wait while we load your content...