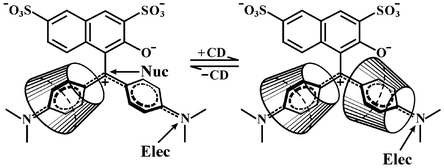
The kinetics of the oxidation of the N,N-dimethylamino substituted triarylmethane dye, Green S, by Caro's acid have been measured over a wide range of pH in the presence, where necessary, of the free radical scavenger, N-tert-butyl-α-phenylnitrone. The rate constants for the reaction of the dianionic form of the dye, D2−, and −OOSO3− is 3.5 × 10−2 dm3 mol−1 s−1. Rate constants for the reactions of D2− and −OOSO3− associated with one, two and three protons, respectively, have been determined, and from UV/visible spectral evidence for electrophilic attack of the peroxide on the tertiary amino group of the dye, assigned to the reactions of D2− and HOOSO3−
(1.8 dm3 mol−1 s−1), HD− and HOOSO3−
(0.41 dm3 mol−1 s−1), and HD− and HOOSO3H (5.9 × 102KC2 dm3 mol−1 s−1, where KC2 is the first acid dissociation constant of HOOSO3H). In contrast, spectral evidence shows that peracetic acid attacks the central carbon of the dye. The effect of β-cyclodextrin on the oxidation of Green S by peracetic acid shows a rate maximum with increasing cyclodextrin concentration. On the other hand, the oxidation by Caro's acid of one of the two tertiary amine nitrogen atoms of the dye is strongly inhibited at the lower concentrations of cyclodextrin and no further inhibition is observed at higher concentrations. The results are interpreted using the transition state pseudo-equilibrium constant approach. They are consistent with pathways involving the binding of both one and two molecules of cyclodextrin to the dye, where the orientation of the cyclodextrin is discussed in terms of simple field effects.

 Please wait while we load your content...
Please wait while we load your content...