A chemoenzymatic synthesis of (−)-hirsutene from toluene
Abstract
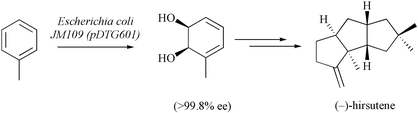
The enantiomerically pure cis-1,2-diol 2, which is obtained by microbial oxidation of toluene, has been converted, via a sequence of reactions including high-pressure promoted Diels–Alder cycloaddition and oxa-di-π-methane rearrangement steps, into the triquinane (−)-hirsutene (1).

 Please wait while we load your content...
Please wait while we load your content...