Synthesis and nicotinic acetylcholine-binding properties of epibatidine homologues: homoepibatidine and dihomoepibatidine
Abstract
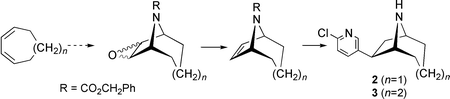
Homoepibatidine 2 and dihomoepibatidine 3 have been synthesised from the 8-azabicyclo[3.2.1]oct-6-ene 8 and the 9-azabicyclo[4.2.1]oct-7-ene 9, respectively, the key precursors for reductive Heck coupling reactions. Alternative routes starting from cyclohepta- and cycloocta-1,3-diene are described; deoxygenation of tropane and homotropane epoxides provides a convenient route to 8 and 9. The enantiomers of 2 show similar potency at nicotinic receptors to the corresponding epibatidine enantiomers; the affinity of 3 is lower.

 Please wait while we load your content...
Please wait while we load your content...