Palladium catalyzed stereocontrolled synthesis of C-aryl glycosides using glycals and arenediazonium salts at room temperature†‡
Abstract
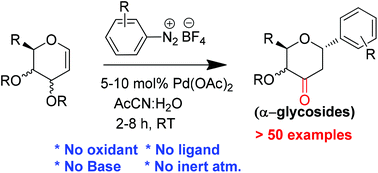
A stereocontrolled synthesis of aryl-C-glycosides was achieved using glycals and aryldiazonium salts in the presence of palladium acetate. A wide range of glycals including D-glucal, D-galactal, L-rhamnal, D-xylal and D-ribal underwent C-arylation at the anomeric carbon in the presence of different aryldiazonium tetrafluoroborates and gave synthetically useful 2,3-deoxy-3-keto-α-aryl-C-glycosides in good to excellent yields. Broad substrate scope, simple operation and room temperature reactions make this protocol very attractive in organic synthesis.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...