A silver catalyzed domino reaction of N-cyanamide alkenes and 1,3-dicarbonyls for the synthesis of quinazolinones†
Abstract
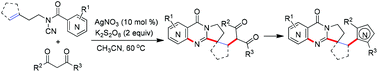
A silver catalyzed domino reaction of N-cyanamide alkenes and 1,3-dicarbonyls including 1,3-diketones and ethyl acetoacetate has been developed for the facile synthesis of quinazolinones. In the presence of AgNO3/K2S2O8, the diketones could be converted to radicals and coupled with N-cyanamide alkenes to undergo a cyclization cascade for accessing quinazolinones. This method features mild reaction conditions, readily available starting materials, and valuable synthetic utility. Moreover, the products could be further transformed into various heterocycles.


 Please wait while we load your content...
Please wait while we load your content...