CoCl2-promoted TEMPO oxidative homocoupling of indoles: access to tryptanthrin derivatives†
Abstract
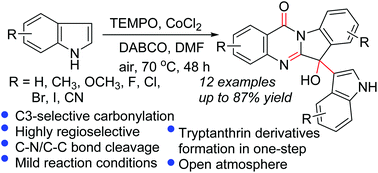
A novel TEMPO/CoCl2-promoted aerobic oxidation of indoles was developed. The reaction provided one-step access to tryptanthrin derivatives in moderate to good yields and excellent regioselectivity via a cascade process. The reactions could be carried out under mild reaction conditions with varying functional group tolerance, especially halogen functional groups. Mechanistic studies disclosed that the oxygen atom in the desired product originated from molecular dioxygen.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...