Palladium catalyzed chloroethoxylation of aromatic and heteroaromatic chlorides: an orthogonal functionalization of a chloroethoxy linker†
Abstract
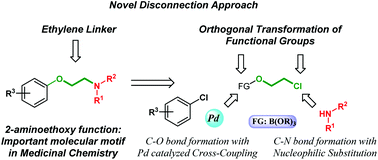
A novel disconnection based on cross-coupling chemistry was designed to access pharmaceutically relevant aryl-aminoethyl ethers. The developed palladium-catalyzed functionalization of aryl- and heteroaryl chlorides with a sodium tetrakis-(2-chloroethoxy) borate salt is orthogonal to the simple nucleophilic replacement of the chloro function of the ethylene linker. The transformation enables efficient 2-chloroethoxylation in the absence of an additional external base. Subsequent amine substitution of the alkyl halide affords 2-aminoethoxy arenes. The applicability of this method was demonstrated through the synthesis of various aryl- and heteroaryl-alkyl ethers, including the intermediates of marketed drug molecules.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...