Bifunctional mannoside–glucosinolate glycoconjugates as enzymatically triggered isothiocyanates and FimH ligands†
Abstract
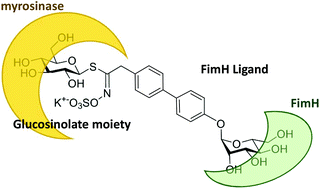
Glucosinolates are sulfur-containing secondary metabolites found in plants of the Brassicale order. They are precursors of isothiocyanate species, resulting from C–S hydrolysis catalysed by the thioglucohydrolase myrosinase. We describe the synthesis of bifunctional glucosinolate–mannoside glycoconjugates combining both the structural features of a substrate of myrosinase and a ligand of the lectin FimH. We show that these glycoconjugates serve as enzyme substrates and that myrosinase can indeed hydrolyze the glucosinolate moiety with affinities (KM, Vmax) comparable to the natural substrates glucomoringin and sinigrin. This enzymatic hydrolysis of the thioglycosidic bond led to the efficient formation of an isothiocyanate which was assessed by the formation of the corresponding dithiocarbamate derivatives. Finally, we show that our synthetic bifunctional glycoconjugates also serve as FimH ligands where the glucosinolate moiety does not hamper the interaction with the lectin. Our findings set the stage for an original bioconjugation tool, allowing for myrosinase-triggered specific labelling of lectins using glucosinolate glycoconjugates as non-toxic, water soluble isothiocyanate precursors.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...