Synthesis of acyloxyl pyrazolines by copper-mediated aminoacyloxylation of unsaturated ketohydrazones†
Abstract
An efficient and step-economical copper-mediated intra-/intermolecular aminoacyloxylation of β,γ-unsaturated hydrazones has been developed. Copper carboxylates serve as both reaction promoters and carboxylate sources in these easily conducted reactions. This method provides straightforward access to diversely useful acyloxyl pyrazolines, which are hard to access traditionally, in good to excellent yields.
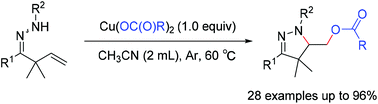
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...