Campestarenes: new building blocks with 5-fold symmetry†
Abstract
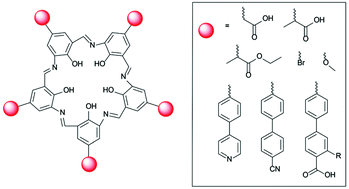
Campestarene is a planar, shape-persistent macrocycle with 5-fold symmetry. A range of derivatives bearing peripheral functional groups suitable for generating supramolecular interactions has been designed and synthesised for potential applications in creating 2D quasicrystal molecular assemblies. The new campestarene derivatives bear ester, carboxylic acid, methoxy, bromo, 4-pyridyl, 4-cyanophenyl and 4-phenyl carboxylic acid groups, including further derivatives of the latter two bearing alkyl chains on the phenyl groups to improve solubility. The campestarene derivatives were prepared by reductive condensation of phenol precursors bearing nitro and formyl groups using Na2S2O4. The target functional groups were installed either by pre-cyclisation derivatisation or by synthesis of methoxy-substituted campestarene and subsequent derivatisation. The cyclisation reaction is tolerant of the functional groups introduced. The ten new campestarene derivatives were characterised by NMR spectroscopy and MALDI-TOF MS, although the poor solubility of some examples precluded their detailed characterisation.
- This article is part of the themed collections: Supramolecular chemistry in OBC and Celebrating excellence in research: women of organic chemistry


 Please wait while we load your content...
Please wait while we load your content...