Comparison of riboflavin-derived flavinium salts applied to catalytic H2O2 oxidations†
Abstract
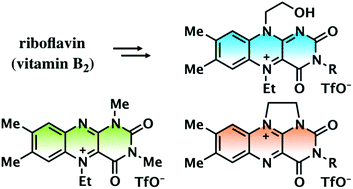
A series of flavinium salts, 5-ethylisoalloxazinium, 5-ethylalloxazinium, and 1,10-ethylene-bridged alloxazinium triflates, were prepared from commercially available riboflavin. This study presents a comparison between their optical and redox properties, and their catalytic activity in H2O2 oxidations of sulfide, tertiary amine, and cyclobutanone. Reflecting the difference between the π-conjugated ring structures, the flavinium salts displayed very different redox properties, with reduction potentials in the order of: 5-ethylisoalloxazinium > 5-ethylalloxazinium > 1,10-ethylene-bridged alloxazinium. A comparison of their catalytic activity revealed that 5-ethylisoalloxazinium triflate specifically oxidises sulfide and cyclobutanone, and 5-ethylalloxazinium triflate smoothly oxidises tertiary amine. 1,10-Bridged alloxazinium triflate, which can be readily obtained from riboflavin in large quantities, showed moderate catalytic activity for the H2O2 oxidation of sulfide and cyclobutanone.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...