A novel approach to sesquiterpenoid benzoxazole synthesis from marine sponges: nakijinols A, B and E–G†
Abstract
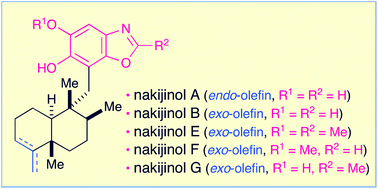
Nakijinols A, B and analogues E through G, which are structurally unique and biologically significant sesquiterpenoid benzoxazoles, can be efficiently obtained in a highly unified manner from the sesquiterpenoid quinone, smenospongine. The starting material is accessible from the (+)-5-methyl Wieland–Miescher ketone. The synthetic method features strategic construction of the requisite dihydroxylated benzoxazole substructure via the ring closure of the N-(2-hydroxyphenyl)-formamide or -acetamide moiety. The synthesis of nakijinols is reported here for the first time. The absolute configurations of nakijinols A and E were also established.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...