The effect of varying the anion of an ionic liquid on the solvent effects on a nucleophilic aromatic substitution reaction†
Abstract
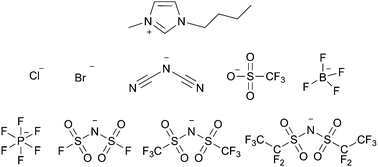
A variety of ionic liquids, each containing the same cation but a different anion, were examined as solvents for a nucleophilic aromatic substitution reaction. Varying the proportion of ionic liquid was found to increase the rate constant as the mole fraction of ionic liquid increased demonstrating that the reaction outcome could be controlled through varying the ionic liquid. The solvent effects were correlated with the hydrogen bond accepting ability (β) of the ionic liquid anion allowing for qualitative prediction of the effect of changing this component of the solute. To determine the microscopic origins of the solvent effects, activation parameters were determined through temperature-dependent kinetic analyses and shown to be consistent with previous studies. With the knowledge of the microscopic interactions in solution, an ionic liquid was rationally chosen to maximise rate enhancement demonstrating that an ionic solvent can be selected to control reaction outcome for this reaction type.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...