Aromatic substitutions of arenediazonium salts via metal catalysis, single electron transfer, and weak base mediation
Abstract
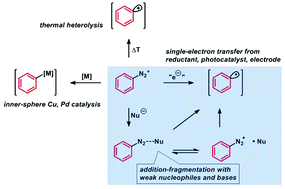
The re-discovery of arenediazonium salts as stable and easily accessible electrophiles for radical aromatic substitutions initiated by single-electron transfer has led to the development of numerous protocols. The diverse set of methods involving metal-mediated, transition metal-catalyzed, photoredox-catalytic and electrochemical electron-transfer mechanisms has recently been complemented by reactions that operate under the mild conditions of very weak inorganic bases. This method provides great advantages with regard to operational simplicity, price, hazard potential, and scalability. The scope of weak base-mediated radical aromatic substitutions has been greatly expanded to include various C–C and C–heteroatom bond forming reactions, cyclizations and rearrangements, and even reactions that preserve the reduction-labile dinitrogen functionality within the product structure. The recent reports of highly fruitful synthetic applications have impressively documented that non-hazardous, inexpensive, and easily accessible inorganic bases can mimic the reducing ability of metal salts, complex reductants, or photo/electrochemical setups. This review covers a concise summary of the most important development in the field and provides detailed analyses of reaction scopes and mechanisms.


 Please wait while we load your content...
Please wait while we load your content...