Vitamin E-based glycoside amphiphiles for membrane protein structural studies†
Abstract
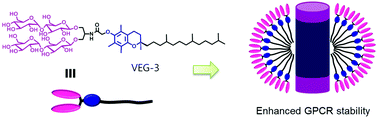
Membrane proteins play critical roles in a variety of cellular processes. For a detailed molecular level understanding of their biological functions and roles in disease, it is necessary to extract them from the native membranes. While the amphipathic nature of these bio-macromolecules presents technical challenges, amphiphilic assistants such as detergents serve as useful tools for membrane protein structural and functional studies. Conventional detergents are limited in their ability to maintain the structural integrity of membrane proteins and thus it is essential to develop novel agents with enhanced properties. Here, we designed and characterized a novel class of amphiphiles with vitamin E (i.e., α-tocopherol) as the hydrophobic tail group and saccharide units as the hydrophilic head group. Designated vitamin E-based glycosides (VEGs), these agents were evaluated for their ability to solubilize and stabilize a set of membrane proteins. VEG representatives not only conferred markedly enhanced stability to a diverse range of membrane proteins compared to conventional detergents, but VEG-3 also showed notable efficacy toward stabilization and visualization of a membrane protein complex. In addition to hydrophile–lipophile balance (HLB) of detergent molecules, the chain length and molecular geometry of the detergent hydrophobic group seem key factors in determining detergent efficacy for membrane protein (complex) stability.


 Please wait while we load your content...
Please wait while we load your content...