Eosin Y-catalyzed, visible-light-promoted carbophosphinylation of allylic alcohols via a radical neophyl rearrangement†
Abstract
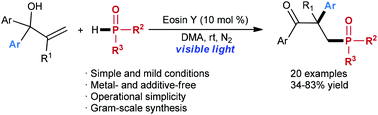
A visible-light-promoted phosphinylation of allylic alcohols with concomitant 1,2-aryl migration is described. This transformation proceeds smoothly under metal-free and mild conditions by using an inexpensive organic dye, eosin Y, as the photocatalyst, affording various β-aryl-γ-ketophosphine oxides in moderate to good yields. Mechanistic studies suggested that the 1,2-aryl migration proceeded through a radical (neophyl) rearrangement.

 Please wait while we load your content...
Please wait while we load your content...