Stereospecific radiosynthesis of 3-fluoro amino acids: access to enantiomerically pure radioligands for positron emission tomography†
Abstract
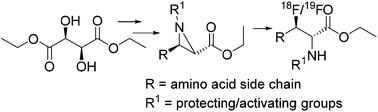
A variety of substituted non-racemic aziridine-2-carboxylates equivalent to amino acids were prepared and subjected to ring opening reaction by [18F/19F]fluoride. The regio and stereospecific ring opening depends on the substituents on the nitrogen as well as both the carbons of aziridines. The applicability of the methods to afford access to 3-[18F/19F]fluoro amino acids are illustrated.

 Please wait while we load your content...
Please wait while we load your content...