Electrophilic fluorination of stereodefined disubstituted silyl ketene hemiaminals en route to tertiary α-fluorinated carbonyl derivatives†
Abstract
A highly diastereoselective synthesis of tertiary α-fluoro carbonyl compounds is reported in only two chemical steps from a simple alkyne through the reaction of stereodefined fully substituted silyl ketene hemiaminal derivatives with Selectfluor.
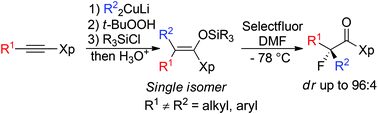
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...