Direct access to spirobiisoxazoline via the double 1,3-dipolar cycloaddition of nitrile oxide with allenoate†
Abstract
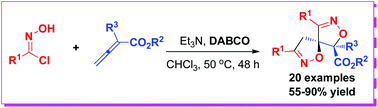
The double [3 + 2]-cycloadditions between nitrile oxides and allenoates have been achieved. In the presence of DABCO combined with Et3N, 2-substituted buta-2,3-dienoates reacted with oxime chlorides to afford spirobiisoxazolines in 55–90% yields via the double 1,3-dipolar cycloaddition. Notably, the construction of double isoxazoline moieties and two chiral centers including a spiro carbon center was achieved.


 Please wait while we load your content...
Please wait while we load your content...