Synthesis of azulene-substituted benzofurans and isocoumarins via intramolecular cyclization of 1-ethynylazulenes, and their structural and optical properties†
Abstract
The preparation of azulene-substituted benzofurans and isocoumarins was established by two types of intramolecular cyclization reaction of 1-ethynylazulenes. 2-(1-Azulenyl)- and 2,3-bis(1-azulenyl)benzofurans were prepared by the palladium-catalyzed cross-coupling reaction of 1-iodoazulenes with 2-ethynylphenol and that of 1-ethynylazulenes with 2-iodophenol under Sonogashira–Hagihara reaction conditions following the intramolecular nucleophilic addition of the oxygen nucleophile to the presumed 1-arylethynylazulenes. In contrast, 1-(phenylethynyl)azulenes bearing an o-methoxycarbonyl function on the substituted phenyl moiety exhibited intramolecular cyclization either in the presence of trifluoroacetic acid or N-iodosuccinimide (NIS) to afford azulene-substituted isocoumarins and 4-iodoisocoumarins, and the structures were clarified by single-crystal X-ray analysis. The optical properties of these compounds were also investigated by UV/vis spectroscopy and theoretical calculations.
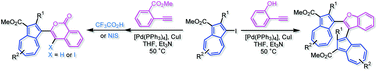


 Please wait while we load your content...
Please wait while we load your content...