Palladium-catalysed stereoselective synthesis of 4-(diarylmethylidene)-3,4-dihydroisoquinolin-1(2H)-ones: expedient access to 4-substituted isoquinolin-1(2H)-ones and isoquinolines†
Abstract
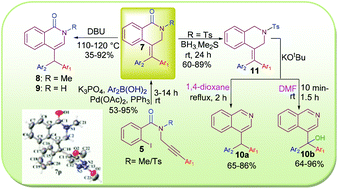
An efficient method has been developed for the stereoselective synthesis of 4-(diarylmethylidene)-3,4-dihydroisoquinolin-1(2H)-ones 7 through tandem Heck–Suzuki coupling at rt using easily available substrates. DBU easily converted the exocyclic double bond of these compounds to endo, furnishing 8 and 9. Reduction of the carbonyl group of 7 was smoothly carried out with borane dimethyl sulphide. Subsequent treatment with KOtBu provided an easy access to 4-substituted isoquinolines 10a if carried out in refluxing 1,4-dioxane, while reaction in DMF at rt led to the incorporation of an extra hydroxyl group at the benzylic position of the isoquinolines to give 10b. This straightforward and metal free procedure would serve as a better alternative to the prevalent procedures. Few of the products could also be transformed into heterocyclic scaffolds structurally resembling known bioactive compounds.


 Please wait while we load your content...
Please wait while we load your content...