Efficient dehydrative alkylation of thiols with alcohols catalyzed by alkyl halides†
Abstract
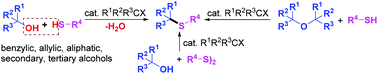
Alcohols can be efficiently converted into the useful thioethers by a transition metal- and base-free dehydrative S-alkylation reaction with thiols or disulfides by employing alkyl halides as the effective catalyst. This simple and efficient method is a green and practical way for the preparation of thioethers, as it tolerates a wide range of substrates such as aryl and alkyl thiols, as well as benzylic, allylic, secondary, tertiary, and even the less reactive aliphatic alcohols.


 Please wait while we load your content...
Please wait while we load your content...