Hydroxylation of anilides by engineered cytochrome P450BM3†
Abstract
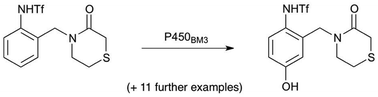
Biocatalytic direct monohydroxylation of anilides has been achieved on preparative scale using mutant cytochrome P450BM3 enzymes. Representative mono- and disubstituted N-trifluoromethanesulfonyl anilides are shown to be converted in most cases to the corresponding 4-hydroxy derivatives, with substituent hydroxylation also occurring in two cases. By mutation variation, it is possible to achieve selective hydroxylation of either ring- or side-chain sites.


 Please wait while we load your content...
Please wait while we load your content...