25 years and still going strong: 2′-O-(pyren-1-yl)methylribonucleotides – versatile building blocks for applications in molecular biology, diagnostics and materials science
Abstract
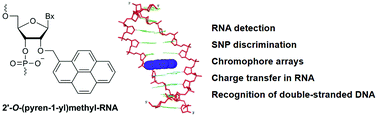
Oligonucleotides (ONs) modified with 2′-O-(pyren-1-yl)methylribonucleotides have been explored for a range of applications in molecular biology, nucleic acid diagnostics, and materials science for more than 25 years. The first part of this review provides an overview of synthetic strategies toward 2′-O-(pyren-1-yl)methylribonucleotides and is followed by a summary of biophysical properties of nucleic acid duplexes modified with these building blocks. Insights from structural studies are then presented to rationalize the reported properties. In the second part, applications of ONs modified with 2′-O-(pyren-1-yl)methyl-RNA monomers are reviewed, which include detection of RNA targets, discrimination of single nucleotide polymorphisms, formation of self-assembled pyrene arrays on nucleic acid scaffolds, the study of charge transfer phenomena in nucleic acid duplexes, and sequence-unrestricted recognition of double-stranded DNA. The predictable binding mode of the pyrene moiety, coupled with the microenvironment-dependent properties and synthetic feasibility, render 2′-O-(pyren-1-yl)methyl-RNA monomers as a promising class of pyrene-functionalized nucleotide building blocks for new applications in molecular biology, nucleic acid diagnostics, and materials science.
- This article is part of the themed collection: Nucleic Acid Modifications


 Please wait while we load your content...
Please wait while we load your content...