An enantioselective synthesis of spiro-oxindole-based 3,4-dihydropyrroles via a Michael/cyclization cascade of 3-aminooxindoles with 2-enoylpyridines†
Abstract
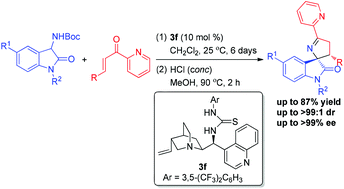
An organocatalytic and highly diastereo- and enantioselective reaction of 3-aminooxindoles with 2-enoylpyridines for the synthesis of chiral spiro[pyrrolidin-3,2′-oxindole] derivatives has been achieved. With the cinchonidine-based thiourea catalyst, the asymmetric Michael/cyclization reaction sequence of 3-aminooxindoles with 2-enoylpyridines followed by the dehydration and deprotection with concentrated hydrochloric acid provided a wide range of chiral 3′,4′-dihydrospiro[indoline-3,2′-pyrrol]-2-ones, bearing two adjacent tri- and tetrasubstituted stereocenters, in moderate to good yields with overall excellent stereoselectivities. The synthetic application of this methodology was presented by the scale-up experiment and transformation of product 5a into the spiro-oxindole-based pyrrolidine 6. Furthermore, a plausible transition state for this cascade reaction sequence was also proposed. And this work will provide a new way to access chiral spiro[pyrrolidin-3,2′-oxindole] derivatives.


 Please wait while we load your content...
Please wait while we load your content...