Iron-catalyzed intermolecular cycloaddition of diazo surrogates with hexahydro-1,3,5-triazines†
Abstract
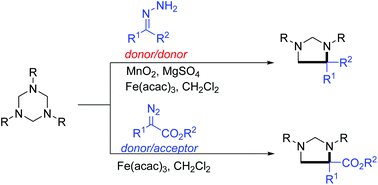
We report here an unprecedented iron-catalyzed cycloaddition reaction of diazo surrogates with hexahydro-1,3,5-triazines, providing five-membered heterocycles in moderate to high yields under mild reaction conditions. This cycloaddition features C–N and C–C bond formation using a cheap iron catalyst. Importantly, different to our former report on a gold-catalyzed system, both donor/donor and donor/acceptor diazo substrates are tolerated in this iron-catalyzed protocol.


 Please wait while we load your content...
Please wait while we load your content...